The Buffalo Neuroimaging Analysis Center (BNAC) is a dedicated preclinical research center that provides a number of preclinical services, including housing and induction of preclinical models, behavioral testing, MRI-PET image acquisition and analysis protocols, immunology and histology examinations.
BNAC ensures fast contracting, Institutional Animal Care and Use Committee (IACUC) approval and start-up processes, and is structured to manage multiple projects with hundreds of preclinical scans.
BNAC brings to every preclinical trial a high degree of professionalism and integrity that stipulates unequivocal respect for deadlines and commitments. BNAC has recently conducted several preclinical trials with currently FDA approved DMTs, as well as those in various stages of preclinical and clinical development, using various preclinical models of multiple sclerosis (MS).
Housing and Induction of Preclinical Models
Preclinical models are permitted to be housed, for long periods of time for longitudinal studies, in the Division of Comparative Medicine and Laboratory Animal Facilities (CM-LAF), which is a centralized service organization at the University at Buffalo that supports animal care, research and education across campus and at the University's affiliated institutions.
Our expertise includes, but is not limited to the following preclinical models:
- Theiler's murine encephalomyelitis virus (TMEV) model of chronic demyelination
- Human (MOG 1-125) and (MOG-35-55) myelin oligodendrocyte glycoprotein-induced experimental autoimmune encephalomyelitis (EAE)
- Cuprizone
- Lysolecithin
As part of BNAC preclinical services we provide:
- Preclinical Models Induction and Immunization
- Intracerebral Injection in Rodents
- Drug Preparation and Administration
- Blind Data Collection and Analysis
- Oral Gavaging
Behavioral Testing
Preclinical models are clinically tested in the Division of Comparative Medicine and Laboratory Animal Facilities (CM-LAF) that is a centralized service organization at the University at Buffalo, which supports animal care, research and education across campus and at the University's affiliated institutions.
As part of BNAC preclinical services we are offering daily/weekly assessments of:
- Body weight
- Clinical Score Monitoring
- Rotarod Test for Motor Skills
- T-CAT Procedure for Evaluation of Cognition
9.4T MRI Acquisition with Cryogenically-Cooled Coil
In collaboration with the Center for Biomedical Imaging, BNAC performs imaging at the 9.4T scanner, which is among the most powerful preclinical MRI scanners available. The Center for Biomedical Imaging is a key core facility of the UB Clinical and Translational Science Institute (CTSI) and the NIH-funded Clinical and Translational Science Award (CTSA). The scanner has expanded our preclinical imaging capacity enormously, enabling imaging into new areas of translational research.
We are dedicated to providing innovative, state-of-the-art, affordable, invasive and non-invasive, high-resolution, in-vivo and ex-vivo imaging on 9.4T MRI. The 9.4 Tesla Bruker BioSpin BioSpec 94/20 USR is a horizontal bore imaging/spectroscopy system that gives us the capability to characterize novel contrast agents and scan live animals or tissue specimens up to 500 grams with an isotropic resolution of down to 25 μm.
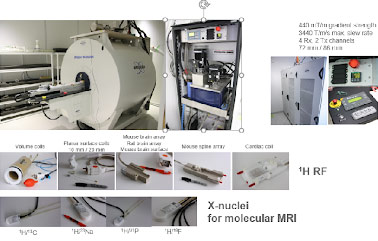
The system is equipped with a variety of coils, including a cryogenically cooled mouse brain coil (Bruker, CryoProbe). The system is also equipped with a rodent isoflurane gas anesthesia system and physiologic monitoring system that can be used for image gating. The instrument can be used to scan various NMR nuclei (X-nuclei) including 1H, 23Na, 31P, 19F and 13C.
T1 and T2 anatomical scans are possible, as is the creation of parametric T1, T2 and T2* maps. The system is also capable of functional MRI (EPI), diffusion weighted (DWI) and diffusion tensor imaging (DTI), localized spectroscopy (STEAM and PRESS) for in vivo metabolite profile characterization, chemical-shift imaging, and perfusion imaging with contrast-based agents.
These specifications allow investigators to visualize and quantify metabolites (MR spectroscopy), anatomical structures, tumor morphology, blood flow/vessels, fiber pathways, drug effects, brain activity and heart motion, quantification of neuro-metabolites including gamma aminobutyric acid (GABA) and glutamate.
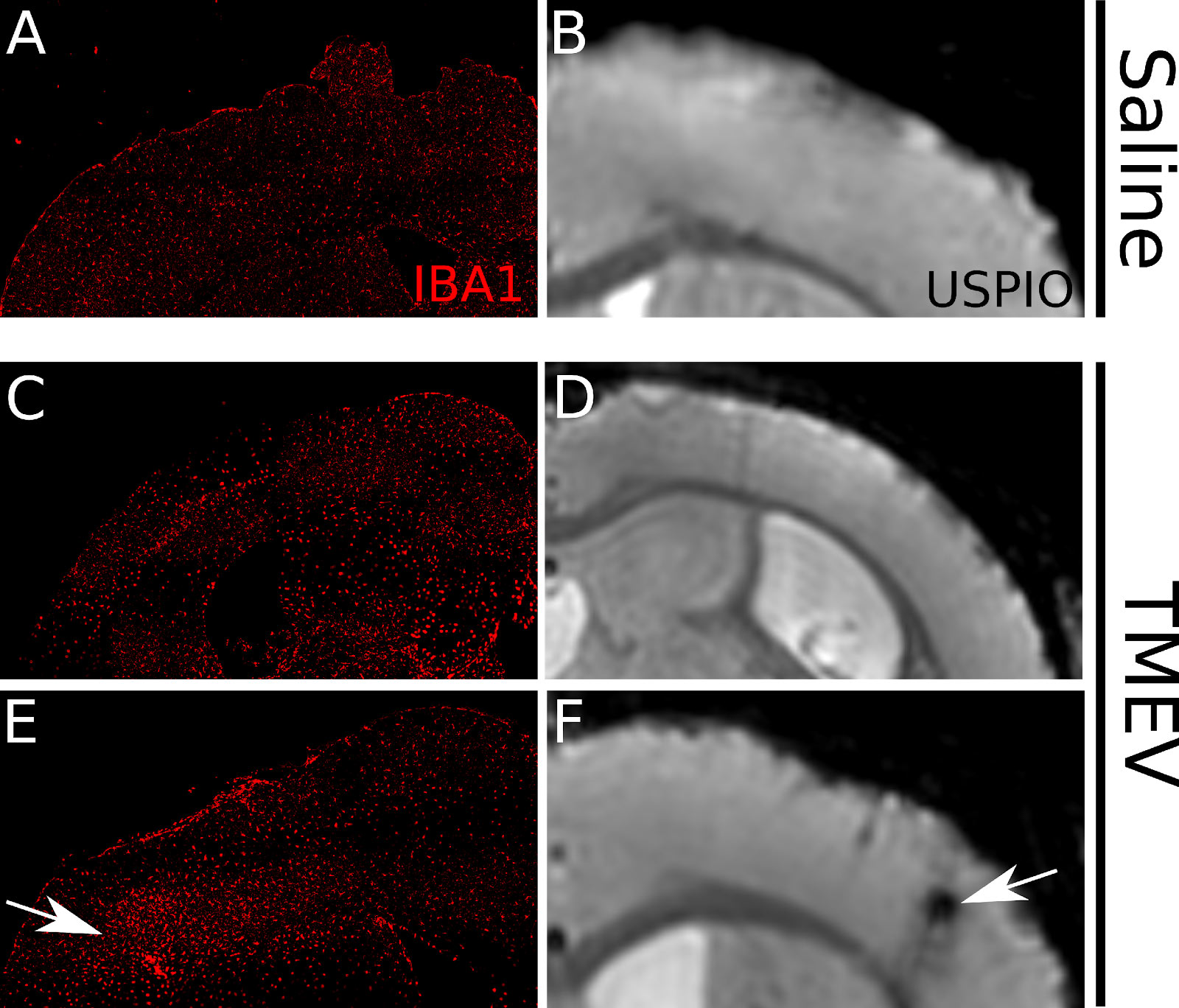
We have set up for imaging of infiltrating immune cells into the mouse brains using high field MRI. Ferumoxytran are ultra-small superparamagnetic iron oxide (USPIO) nanoparticle contrast agent (CA) that is dextran-coated iron oxides. Innate immune cells, such as macrophages or neutrophils internalize the USPIO and carry it to the sites of pathology in the central nervous system (CNS). These act as optimal agents for visualizing the temporal profile of neuro-inflammation in CNS diseases, such as MS. We have optimized and validated the detection of infiltrating immune cells within CNS tissue via USPIO enhanced imaging both in MOG-EAE and TMEV.
BNAC Preclinical Core lab developed an MRI sequence, to track EAE disease progression using FLAIR-RARE gadolinium-based scanning protocol on an ultra-high field 9.4T small animal scanner. This allows for the imaging of promising biomarker, the leptomeningeal contrast enhancement (LMCE). The study characterized pattern of LMCE using serial MRI in EAE model of MS, and its association with clinical symptoms and disease progression. Study also characterized the relationship between LMCE MRI scan results and the underlying pathology associated with LMCE scan intensity. The work showed that EAE brains exhibit retention of gadolinium at early sites of inflammation and associate with high inflammatory cell density. This showed that LMCE imaging can trace EAE disease progression and help understand immune system modulating therapies, especially for assessing treatment’s effects in early disease state.
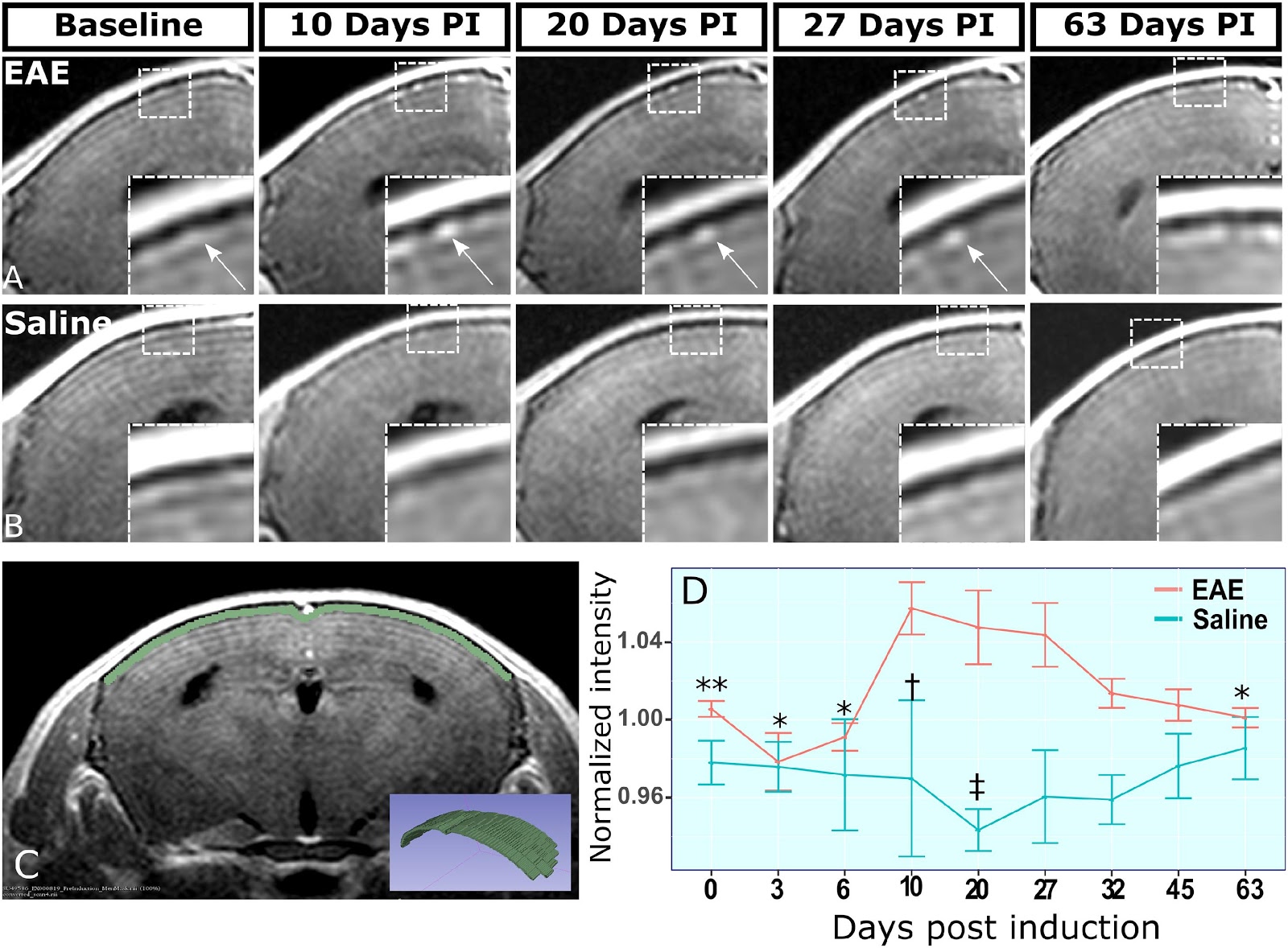
(A) shows representative stage matched slices from EAE-MOG animal brain scans with peak gadolinium-based signal during early disease state. The arrow points at nodule like structure which commonly appeared at early phase of the disease and remained stable through the disease course. (B) shows control animal brain scans at corresponding timepoints. Inset is showing enlarged view of the meninges. (C) shows the placement of 4 voxel wide region of interest at the dorsal meninges of brain on 5 consecutive slices spanning the mid forebrain. The inset is a 3D rendering of the region of interest. (D) shows graphs the voxel-wise mean signal intensity within the region of interest for each animal within the study. * p < .05 and ** p < .01, Students t-test for comparison with 10 dPI EAE signal. † p < .05 and, ‡ p < .01, Students t-test for EAE vs saline comparisons at matching timepoints. Error bars: Standard error of the mean.
Combined MRI and PET Acquisition
Positron emission tomography (PET) and its implementation in patient care have evolved rapidly with the development of new target-specific radiotracers for cancer and neurodegenerative diseases. Small animal PET instruments are being used increasingly to translate novel molecular radioactive tracers from bench to bedside, facilitating diagnosis and testing of new therapeutic interventions. Combining PET with MRI in research in preclinical models enables simultaneous structural and functional information, without additional measurement time. This unique confluence of two powerful imaging modalities represents a major advance in diagnostic imaging. However, simultaneous PET and ultra-high field 9.4 Tesla MRI imaging are not yet commercially available. In partnership with the New York-based startup SynchroPET Inc., Center for Biomedical Imaging developed a miniature prototype PET detector that can be retrofitted into most MRI systems and integrates the anesthesia and vital signs monitoring systems. This setup enables simultaneous PET and MRI acquisition, a significant advance in translational imaging. We have developed radiation safety protocols, experimental workflows, and set up of the procedure room in the Center for Biomedical Imaging. The first pilot project in Krabbe disease has been recently presented.
Post-Mortem Samples Acquisition
The use of post-mortem imaging is expanding throughout the world with increasing use of advanced imaging techniques, such as MRI, that can provide true volumetric information. The questions asked of post-mortem imaging are complex and can be very different. Post-mortem imaging is different to clinical imaging regarding both the appearance of pathology and the information required, but there is much to learn from many years of clinical research in the use of these techniques. Furthermore, it is possible that post-mortem imaging research could be used to conduct clinically relevant research.
In collaboration with the Center for Biomedical Imaging, BNAC is imaging at the 9.4T scanner post-mortem samples in multiple sclerosis (MS) and other neurodegenerative disorders, which allows scanning of tissue specimens up to 500 grams with an isotropic resolution of down to less than 25 μm using cryogenically-cooled coil. The Center for Biomedical Imaging is a key core facility of the UB Clinical and Translational Science Institute (CTSI) and the NIH-funded Clinical and Translational Science Award (CTSA). The scanner has expanded our post-mortem imaging capacity enormously, enabling imaging into new areas of translational research.
Advanced MRI Technology
To facilitate analysis and sharing of imaging data from the 9.4T scanner, we developed a semi-automated reconstruction system for imaging data, in collaboration with the Center for Biomedical Imaging. The system allows a highly efficient, standardized offline image and spectroscopic reconstruction and analysis of raw preclinical imaging data. An example is development of QUASAR, a novel susceptibility mapping approach that yields improved susceptibility maps and decouples non-susceptibility phase contributions, providing valuable information on macromolecular tissue composition including myelin integrity.
Examples of advances in MRI technology developed specifically in response to the needs of internal and external entities working with BNAC and the Center for Biomedical Imaging are:
- post-contrast FLAIR-RARE protocol for detection of leptomeningeal inflammation in preclinical model of MS
- mapping analysis software for the characterization of MRI properties in liquid samples
- post mortem MR microscopy experimental protocol for preclinical models as well as a landmark-based non-linear deformation for comparison of anatomical structures with resolutions below 27 μm
- high resolution 3D imaging sequence that provides a clear 3D visualization of the larger lung vasculature
- development of functional MRI and connectome sequences
- accelerated compressed sensing 3D under sampling sequence
- gradient echo pulse sequence to detect thalamic calcification in concussion
- MEGA-PRESS and HERMES pulse sequences
- development of magnetization transfer protocol
- development of algorithm for reduction of Gibbs ringing in MRI
- use of susceptibility for better characterization of post-concussion syndrome and stroke
- use of cryoprobe for thoracic/lumbar spine imaging by positioning the animal feet-first
- optimized cardiac imaging pulse sequence to assess function and perfusion ultra-fast imaging
- cardiac sequence for tissue characterization of myocardial scar and high-risk peri-infarct region
- sequence for dynamic contrast enhancement that allows characterization on a voxel-per-voxel basis kidney filtration performance in acute kidney failure
- development of Zebra fish and rabbit imaging
Fluorescent and Confocal Laser Scanning Microscopy
The Buffalo Neuroimaging Analysis Center (BNAC) is a dedicated preclinical research center that provides a number of histological preclinical services, including:
- Perfusion-Fixation of Tissue for Histology
- Live Biomaterials Collection and Processing
- Cryostat Sectioning
- General Histology Staining Methods
- Histology Image Analysis
- Histology Image Acquisition Using Wide-field Fluorescent Microscopy
- Histology Image Acquisition Using Confocal Microscopy
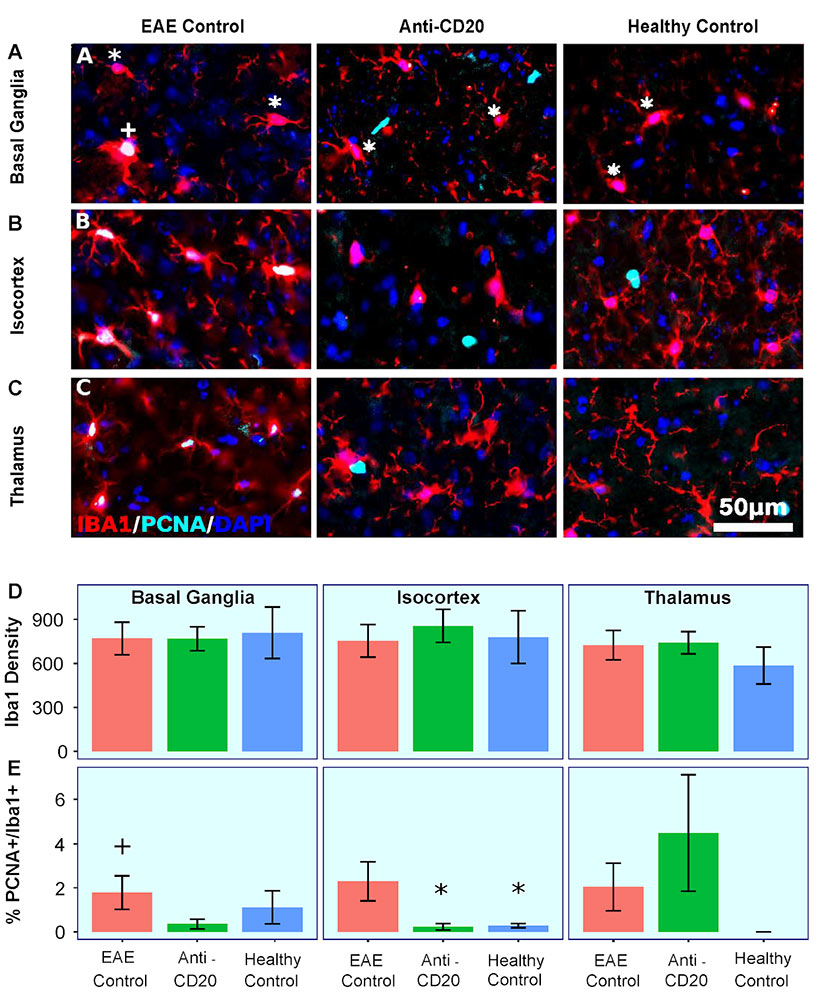
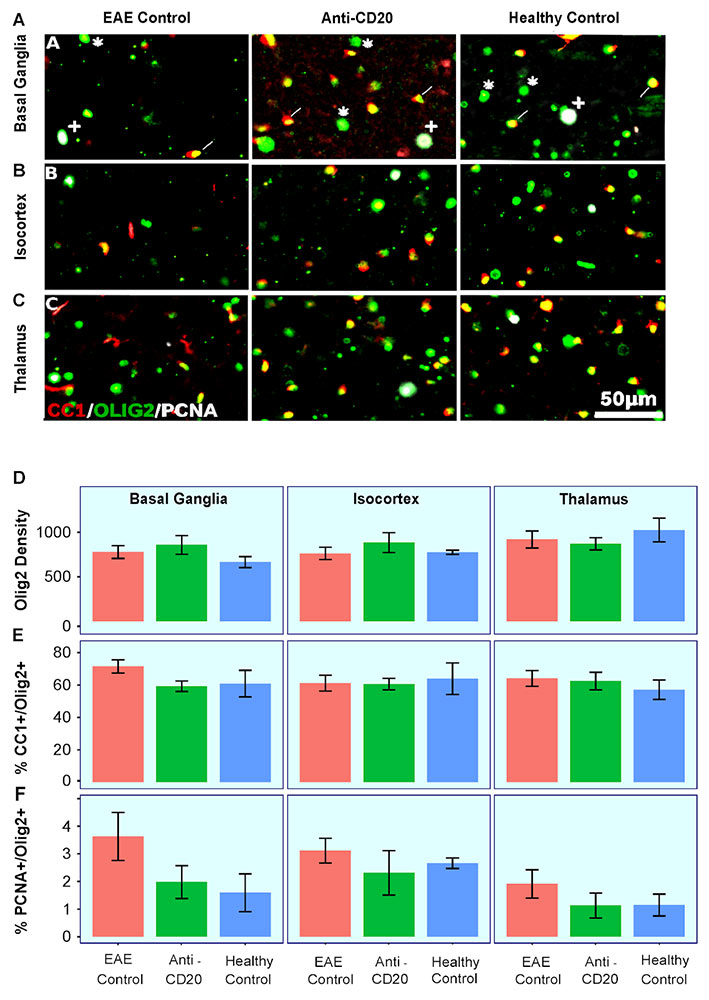
Immunology Examinations
The Buffalo Neuroimaging Analysis Center (BNAC) is a dedicated preclinical research center that provides a number of immunology preclinical services, including:
- RNA Isolation with EZRNA Kit
- qPCR Procedure
- ELISA Analysis for Cytokine Concentration and Antibody Detection
- Flow Cytometry Analysis of Peripheral Blood Mononuclear Cells (PBMCs) to Measure Various Cell Counts

